Orbital diagrams — overview & examples Orbital molecular diagram cl2 s2 molecule bond orbitals electron unpaired bonding c2 energy theory valence electrons paramagnetic molecules diatomic atom Molecular orbital diagrams simplified – megan lim – medium
2.7 Molecular Orbital Theory – Inorganic Chemistry for Chemical Engineers
Orbital molecular theory do diagram combined atoms different two when higher tell energy which inorganic hcl closed ago years so Orbital molecular diagram shown figure solution Orbital molecular diagrams orbitals order simplified medium ne difference note
Diagram n2 mo orbital molecular diagrams electrons chemistry electron lie together two explain determined stack sponsored links via
Orbital molecular theoryOrbital molecular molecules diagram orbitals diatomic bonding of2 delocalized bond atomic libretexts electrons chem correlation hybridization atoms np homonuclear pageindex Orbital molecular diagram chemistry theory draw two mo energy bond o2 order electrons shown ca diagrams oxygen bonding unpaired sigmaAtomic orbitals.
Can someone explain how do i set up a molecular orbital diagram?Orbitals molecular orbital atomic educator diagrams configuration Orbitals molecular bonding orbital theory atomic diatomic delocalized antibonding atoms mo libretexts formation adjacent np molecules internuclear formed readings chemMolecular orbital theory.

4.2.1: molecular orbitals
Orbital molecular o2 energy orbitals diagram order electron atomic diagrams oxygen bond libretexts lecture higher extra chemistry configurations bo theory4.9: molecular orbitals Inorganic chemistryOrbital sulfur monahan caroline.
Orbital molecular diagrams molecules origins chemistry mathematics gif does electrons numbersMolecular orbitals orbital diagram mixing libretexts diatomic formed interactions assuming pageindex row elements second figure Orbital molecular explain socratic exatinSolved: the molecular orbital diagram of no shown in figure 10.47.

How do i write the electron configuration of an oxygen molecule?
Chapter 6.6: polyatomic systems, multiple bonds, resonanceSolved complete this molecular orbital diagram for cn then Understanding molecular orbital theoryMo orbital molecular oxygen orbitals o2 bond diagram configuration theory order paramagnetic electron draw molecule electrons energy diagrams unpaired two.
2.7 molecular orbital theory – inorganic chemistry for chemical engineersCn molecular orbital diagram bond order problem complete mo orbitals determine solved shown note 1s atomic then transcribed text been Bonding molecular bonds orbital polyatomic ethylene resonance libretexts diatomic occupiedChemical forums: molecular orbital diagram.

Lecture extra ii: molecular orbitals with higher energy atomic orbitals
Molecular orbital theoryPhysical chemistry 9.3: molecular orbital theoryChemistry molecular orbitals orbital diagram bonding energy level edu wave two h2 theory atomic bond molecule chemwiki function each atom.
Excited orbital molecular n2 state ground dinitrogen cation so diagram configuration theory molecule electron explain sigma states why chemistry mathrm .


Orbital Diagrams — Overview & Examples - Expii

9.3: Molecular Orbital Theory - Chemistry LibreTexts
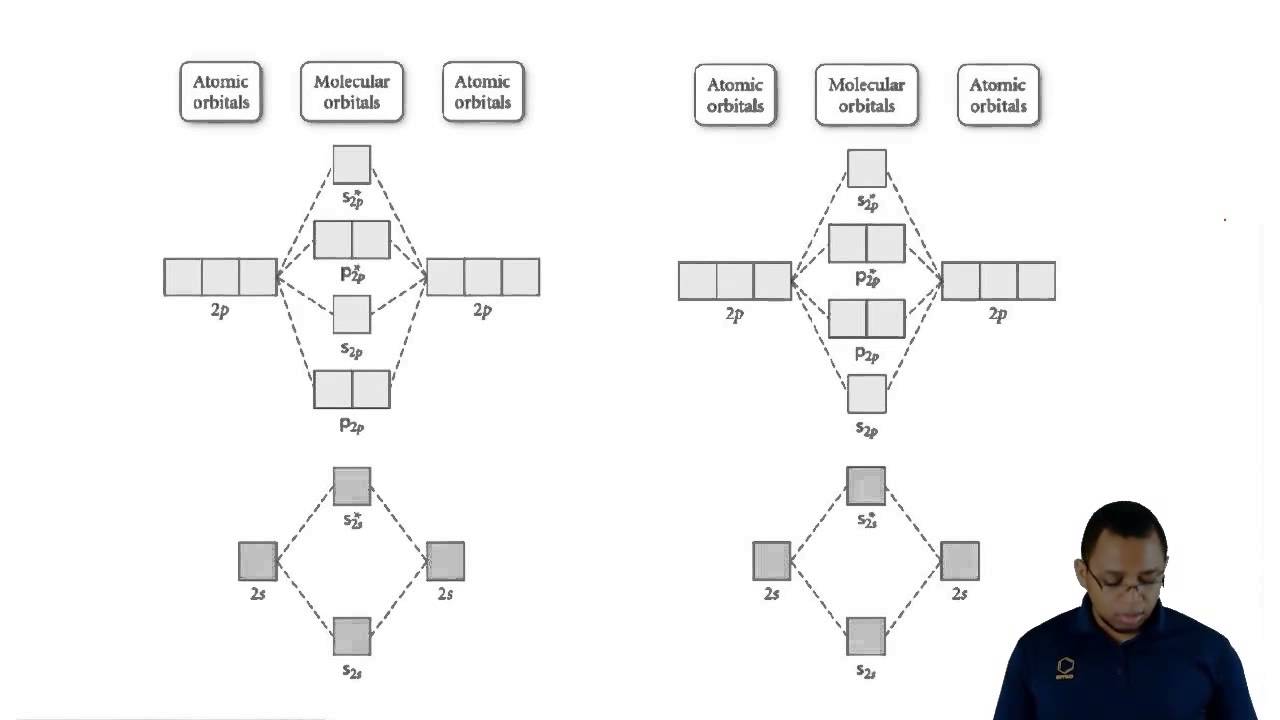
Understanding Molecular Orbital Theory - YouTube

Atomic Orbitals - Educator.com

2.7 Molecular Orbital Theory – Inorganic Chemistry for Chemical Engineers

inorganic chemistry - In molecular orbital theory when two different

4.2.1: Molecular Orbitals - Chemistry LibreTexts

4.9: Molecular Orbitals - Chemistry LibreTexts